1Department of Virology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh; 2Department of Virology, Dhaka Medical College, Dhaka, Bangladesh; 3Department of Gynae & Obstetrics, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
 10.3329/bmrcb.v45i2.42536
10.3329/bmrcb.v45i2.42536  0000-0002-5976-0122
0000-0002-5976-0122
Background: Cervical cancer is one of the most common types of cancer affecting women worldwide. Persistent HPV infection plays a major role in cervical cancer. The risk of cervical cancer has increased in parallel with the incidence of certain genotypes of human papilloma virus (HPV).
Methods: The study was aimed to detect the distribution of HPV genotypes among cervical cancer patients from a specialized hospital in Dhaka, Bangladesh. HPV DNA testing was done by polymerase chain reaction (PCR) using SPF-10 broad-spectrum primers followed by genotyping by reverse hybridization using the INNO-LIPA genotyping system at the Department of Virology, Bangabandhu Sheikh Mujib Medical University, Dhaka.
Results: HPV 16 was more prevalent (72.0%) in cervical cancer patients from Bangladesh followed by type 18 (6.0%) and 45 (2.0%). Genotype 16 and 18 alone and as co-infection were detected in 88.0% cases. Multiple HPV infection was found in 20% patients. Along with high risk (HR) HPV 16, 18 and 45 HR HPV 56, 39, 31 and 58 were also prevalent in multiple infections.
Conclusion: Thus the study concluded that HR HPV 16 and HPV 18 were more prevalent genotypes among cervical cancer patients in a specialised hospital in Bangladesh. Along with HR HPV 16 and HPV 18, HR HPV 45, 56, 39, 31 and 58 were also prevalent.
Keywords: HPV, High risk, genotype, Prevalence, Bangladesh, LIPAGlobally cervical cancer remains the fourth-most common cause of cancer and the fourth-most common cause of death from cancer in women.1 About 99% cases of cervical cancer is associated with HPV infection.2 More than 200 HPV genotypes are reported,3 of which approximately 15 genotypes are classified as oncogenic or high-risk (HR) types,2,4 and are significantly associated with progression to invasive cervical cancer (ICC). HR-HPV DNA is detected in almost all ICC cases.5 The risk of cervical cancer has increased in parallel with the incidence of HR HPV, therefore, the presence of these genotypes indicate a significant risk factor for the development of cervical cancer.6,7
Epidemiologically, significant geographic variation in distribution of HPV genotypes have been reported.8-10 Although region to region or country to country distribution of HPV genotypes vary, HR HPV 16 and 18 are the most prevalent genotypes worldwide among cervical cancer patients followed by 31,33,45,52,58.11,12 A population based study from Bangladesh reported that HPV 16, 66, 18, 45, 31, 53 are the prevalent genotypes but other HR-HPV types including HPV33, HPV35, HPV52 and HPV58 are also prevalent in the general population.13 However, limited data is available from Bangladesh regarding the prevalent HPV genotypes among cervical cancer patients.
Knowledge of HPV genotypes associated with cervical cancer may contribute to the assessment of the impact of current HPV vaccines to prevent future incidence of cervical cancer and assist in decision making of future preventive strategies. This also serves as a guide to determine what other types should be included in future vaccines to provide maximum protection. In addition, knowledge of genotype distribution in cervical cancer is important to assess the HPV-based screening strategies for prevention of cervical cancer. This study was designed to determine the distribution of HR HPV genotypes among cervical cancer patients of Bangladesh and to identify the most prevalent HR HPV genotypes associated with cervical cancer in Bangladesh.
This cross-sectional study was conducted during January 2014 to June 2014 at the Department of Virology, Bangabandhu Sheikh Mujib Medical University. Specimens were collected from cervical cancer patients of National Institute of Cancer Research & Hospital (NICRH) at Mohakhali, Dhaka and Laboratory study was carried out at the Department of Virology, Bangabandhu Sheikh Mujib Medical University, Shahbag, Dhaka.
A total of 110 histopahologically diagnosed invasive cervical cancer patients who were admitted at the National Institute of Cancer Research & Hospital (NICRH) at Mohakhali, Dhaka were approached and briefed about the study. Among them 100 patients agreed to participate in the study. From these 100 patients, every odd number patient was recruited in the study. A pretested data collection sheet was used as data collection instruments. After taking informed written consent, data was collected from fifty patients by face to face interview by trained research assistant under supervision of the investigators. Information about socio-demographic and reproductive health characteristics, and histopathological examination findings were collected by trained research assistant and recorded in the data collection sheet. After examining the cervix by cuscos speculum cervical samples were collected using the cervical cytobrush, transported and processed using a specially designed cervical sampler containing liquid transport media (Digene HC2 DNA collection device, Qiagen, GmbH, Germany). The samples were stored at -20ºC upon receipt at the laboratory and tested within three months according to the storage guideline (Digene HC2 DNA collection device, Qiagen, GmbH, Germany). HPV DNA testing was done by polymerase chain reaction (PCR) using SPF 10 primers, followed by genotyping by reverse hybridization using the INNO-LIPA genotyping system.
INNO LiPA HPV detection and genotyping: DNA extraction: DNA was extracted by a commercially available kit (Sacace DNA- sorb-A DNA extraction kit, Sacace Biotechnologies Srl, Italy, REF- k-1-1/A) according to the manufacturer’s instructions. DNA concentration was measured in ng/μl by spectrophotometer (Nanodrop 2000 UV-Vis spectrophotometer) measured at the ratio of absorbance at 260 and 280 nm.
PCR amplification of HPV DNA: Broad-spectrum HPV DNA amplification was performed using a short-PCR-fragment (SPF) assay (InnoLIPA HPV genotyping Extra Amp, Fujirebio Europe N.V., Belgium, Lot No: 241431). This assay amplifies a 65-bp fragment of the L1 open reading frame using SPF 10 primers. The SPF10 PCR system was used in a final reaction volume of 50 µl containing 10 µl of the isolated DNA sample and 40µl of the PCR mixture, which contained 2.0 mM MgCl2, 1.5 Units AmpliTaq Gold DNA polymerase, 200 μM each dNTP (dATP, dCTP, dGTP, and dTTP), 10 µl primer mix and distilled water up to 40 μl. This reaction mixture was preheated for 9 min at 94°C for activation of AmpliTaq Gold, and amplified for 40 cycles of 30 s at 94°C for denaturation, 45 s at 52°C for annealing, and 45 s at 72°C for extension, with a final extension of 5 min at 72°C. Appropriate negative and positive controls was used to monitor the performance of the PCR method in each experiment.
HPV genotyping: The INNO-LiPA HPV Genotyping (InnoLIPA HPV genotyping Extra, Fujirebio EuropeN.V., Belgium, Lot No: 400406) was based on the principle of reverse hybridization. 65-bp fragment of the L1 open reading frame of HPV genome was amplified using SPF 10 primers and the resulting biotinylated amplicons were denatured and hybridized with specific oligonucleotide probes. An additional primer pair for the amplification of the human HLA-DPB1 gene was added to monitor sample quality and extraction. The oligonucleotide probes that recognized 28 different HPV genotypes were immobilized as parallel lines to membrane strips. After hybridization and stringent washing, streptavidin-conjugated alkaline phosphatase was added, which binds to any biotinylated hybrid previously formed. Incubation with BCIP/NBT chromogen yields a purple precipitate and the result was visually interpreted using the reference guide provided.
Fifty cervical cancer patients were between 30 to 67 years old, with mean age of 46.22 ± 1.83 yrs. Most of the patients (82.0%) were within 40 to 60 years of age. 58.0% of the patients had no formal education, 12.0% patients could only sign their name, 28.0% had primary education, and only 2.0% had secondary education. Most women came from rural areas and belonged to lower and lower middle socioeconomic class (62.0%) (table I).
| Characteristics | Categories |
Freq |
Age |
30-40yrs |
6 (12.0) |
Demographic area |
Rural area |
43 (86) |
Socioeconomic condition |
Lower class (less than Tk 5000) |
18 (36) |
Religions |
Islam |
49 (98) |
Women’s Education |
No formal education |
29 (58) |
Women’s Occupation |
Housewife |
50 (100) |
84.0% reported 4 or more births. 46.0% patients had their first coitus at 11-15 years of age and 54% at 16-20 years age. The mean age at first coitus was 16.54 ± 0.503 years. 54.0% had used oral contraceptives and 26% gave history of chewing betel nuts and/or tobacco leaves (table II).
| Characteristics | Categories |
Freq (%) |
|
Age of first coitus |
11-15 years |
23 (46) |
|
Number of pregnancies
|
One |
01(02) |
|
Contraceptive history |
Oral contraceptive |
27 (54) 23 (46) |
|
Number of marriage of women |
One |
49 (98) |
|
Number of marriages of husband |
One |
45 (90) |
|
Hisotry of tobacco leaves/betel nuts chewing) |
Yes |
13 (26) |
|
Pathological examinations showed that 84.0% had squamous cell carcinoma (SCC) and 16.0% had adenocarcinoma (ADC) (figure 1).
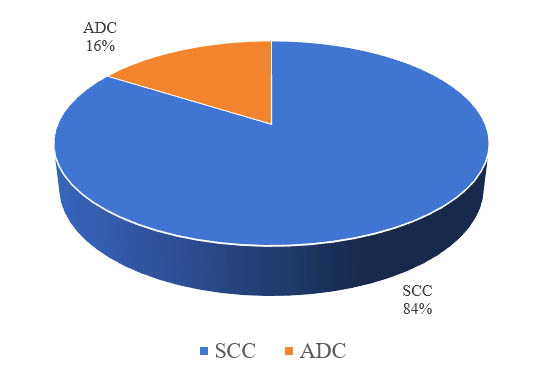
Figure 1: Histopathological diagnosis of cervical cancer patients
HPV DNA was positive in all (100.0%) the cervical cancer patients in this study. It was observed that genotype 16 was more prevalent in cervical cancer patients followed by type 18. HPV 45 was the third prevalent genotype (table III). HPV16 and HPV 18 infection occurred either as a single infection or multiple infection in 88.0% and 16.0% cervical cancer patients respectively. 72.0% of the patients were positive for HPV16 and 6.0% for HPV 18 alone (figure 2).
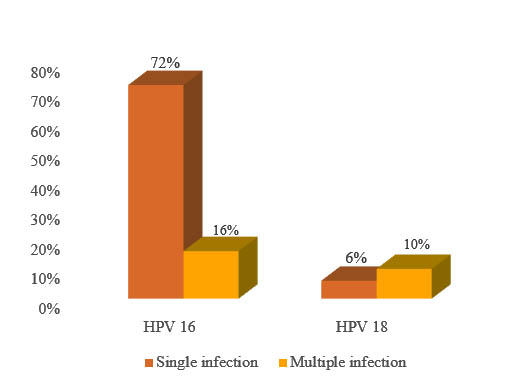
Figure 2: Prevalence of HPV 16 and HPV 18 in single and multiple infection
Genotype 16 and 18 alone and as co-infection were detected in 88.0% cases. Multiple HPV infections were found in 20.0% patients. Along with HR HPV 16, 18 and 45, HR HPV 56, 39, 31 and 58 were also prevalent in multiple infections. Among the multiple infected patients, dual infection with HPV 16/18 was detected in 10.0% and HPV 45/39 in 4.0% cases. Triple infection with HPV 16/45/56 was observed in 4.0% and quadruple infections with HPV 16/31/56/58 were also observed in 2.0% cases (table III).
HPV Genotype |
No. of cases |
Percentage |
Single infection |
||
16 |
36 |
72.0 |
18 |
3 |
6.0 |
45 |
1 |
2.0 |
Multiple infection |
||
16,18 |
5 |
10.0 |
45,39 |
2 |
4.0 |
16,45,56 |
2 |
4.0 |
16,31,56, 58 |
1 |
2.0 |
Total |
50 |
100.0 |
Persistent cervical infection with high-risk HPVs is necessary for the development of cervical cancer; indeed, some epidemiologic data have shown that nearly 100.0% of patients with cervical cancers were positive for HPV.14 In this study, HPV DNA was positive in all the cervical cancer patients. The presence of HPV DNA in 100.0% of cervical cancer patients from Bangladesh were also detected in other studies.15,16 Current study observed that multiparity, young age at first coitus, long term contraceptive use, low socioeconomic status, low education level, betel nut/tobacco leaves chewing etc. may be associated with cervical cancer in Bangladesh. Similar findings among cervical cancer patients were also observed by Nahar Q et al in 2014, Khatun S et al in 2009, Rahman T et al in 2013 from Bangladesh.13,16,17 In this study, the prevalence of SCC was 84.0% whereas ADC was 16.0% (figure 1).
High prevalence of SCC has also been reported from India, they observed 94.2% SCC and 4% ADC among 278 cervical cancer patients. Another study from India, 2014 reported 94.55% SCC and 5.15% ADC among 97 cervical cancer patients.18,19 High prevalence of SCC (96.1%) also observed in Pakistan on 2016.20
Current study observed that the prevalent genotypes of HPV among cervical cancer patients in Bangladesh were HPV16, 18, 45, 56, 39, 31 and 58 either as single or multiple infections. Although, less prevalent HPV56 and HPV 39 were detected in this study, but the more prevalent HPV 33 and HPV 52 were not detected. Although region- or country-specific differences with respect to individual HPV genotypes exist, HPV 16, 18, 31, 33, 45, 52, 58 are responsible for approximately 90.0% of cervical cancers in the world.11,12 HPV 16, 18, 51, 45, 35, 66, 68, 44, 43, 42, 58, and 52 were the prevalent genotypes among the cervical cancer patients at Eastern India reported on 2017.19 A study from Varanasi and adjoining regions of India in 2014 reported that HR HPV 16, 18, 31, 33, 35, 45, 58 and 86 were observed among cervicitis, CIN1/2/3 and cervical cancer patients.21 Another study from Pune India reported on 2012 that the high risk HPV types in declining order of prevalence included HPV16, 56, 18, 39, 35, 51, 31, 59, 33, 58, 68, 45 and 52 in patients with cervical intraepithelial neoplasia among HIV infected women.22 A meta analysis of nine studies from India in 2008 showed that HPV16 was the predominant type (64.8%) among ICC cases, followed by HPV18, 45, 33, 35, 58, 59 and 31.23 In Pakistan the prevalent genotypes in cervical cancers were HPV 16,18,45,56,73,31,52 reported by Loya et al.20 However, the distribution of HPV genotypes is different in China. Chen et al reported that HPV 16 was the highest prevalent genotype, followed by HPV 58, 52, 18 in Chinese women with abnormal cytology from Zhejiang Province between 2011 and 2015.24 Another study from China reported on 2016 that HPV16 was more prevalent in cervical cancer patients followed by HPV 18, 58, and 52.24,25 Genotypes 52 and 58 have generally been reported to be more common in Asia than in other regions of the world.26,27 In India, the prevalence of HPV 52 and 58 appears to be lower compared to other Asian countries.27-29 Our study detected HPV 58 in a single case of multiple infection but HPV 52 was not detected from any case. In this study, multiple HPV infection was found in 20.0% cervical cancer patients. Studies from different countries also reported multiple HPV infection in different cervical lesions.30,31
The present study showed that genotype 16 (72.0%) was more prevalent among Bangladeshi cervical cancer patients followed by HPV 18 (6.0%) and 45 (2%). HPV 16 and 18 alone and as co-infection were detected in 88.0% cases. In 2015 another study from Bangladesh also reported high prevalence of HPV 16 (80.0%) followed by HPV 18 (10.0%) and HPV 45 (6.67%) among cervical cancer patients.15 According to WHO/ICO center, Summary Report 2017, prevalence of HPV 16 and/or HPV 18 among women with cervical cancer is 80.3% in Bangladesh.32 This high prevalence of genotype 16 and 18 is also observed in other countries of Southeast Asia. HPV 16 and 18, either alone or in association with each other, accounted for 73.9% cases in South India, 78.3% in North India, 76.1% in East India and 77.3% in Central India.18 A study from India reported in 2017 that HPV16 (83.78%), HPV 18 (21.08%) were prevalent among cervical cancer patients in Odisha, Eastern India.19 Srivasta et al (2014) observed HPV 16 and HPV 18 together comprise of 79% of the total HPV infection among patients with SCC from Varanasi, India in 2014.21 In another study, it was observed that the estimated HPV-16/18 positive fraction was 78.9% in women with ICC (87.7% in North and 77.2% in South India).23 Loya et al observed that HPV16 (67.3%) was the most detected genotype in Pakistan, followed by HPV 18 (10.2%) and HPV 45 (7.3%).20 That study also reported a significantly higher contribution of HPV16/18 (78.4%) among the ICC cases in Pakistan. The prevalence of HPV16 infection in South Karachi has been reported to be associated with 75.0–93.0% of carcinomas, whereas, HPV18 infection was associated with 4–6.6%.33,34 Studies from other countries with similar religious background have also reported a high prevalence of HPV16 infection in cervical lesions. In Iran, the prevalence was 75% to 76%, and in Saudi Arabia it was 65.2% with a low HPV18 prevalence.35-37
Globally, approximately 70.0% of cervical cancers are attributable to genotypes 16 and/or 18, which may be prevented by first-generation HPV vaccines, Gardasil® and Cervarix®.38 Bangladesh has introduced HPV vaccine against HPV 16 and HPV 18 on 18 May 2016 for the first time by the Ministry of Health, with support from the Global Alliance for Vaccines and Immunizations (GAVI). This new vaccine introduction program has been running for two years in Gazipur district.39 The presence of HPV 16 and 18 in 88% cervical cancer patients in this study indicate that the introduction and implementation of current vaccine against HPV16 and HPV18 by the Ministry of Health, with support from GAVI will reduce the bulk of cervical cancer lesions in Bangladeshi women from Gazipur district. Successful implementation of this vaccination program will help us to adopt a national policy for countrywide vaccination coverage.
Conclusion
This study concluded that HPV16 and 18 are the most prevalent genotypes (88.0%) in cervical cancer patients from Bangladesh. In addition to HPV 16 and 18, this study also detected other high-risk HPV including HPV 45, 56, 39,31 and 58 in cervical cancer patients. In addition to these prevalent genotypes other HR HPV genotypes may be prevalent in cervical cancer patients, thus further studies on HPV genotyping in the wider population is greatly required.
Conflict of interest: There is no conflict of interest about this article.
Acknowledgement
This study was funded by BMRC under Health, Nutrition and Population Sector Programme [HNPSP], Research & Development (Health).
References
- World Health Organization: World Cancer Report.2014. pp. Chapter 5.12. ISBN 9283204298
- Parkin DM, Bray F. The burden of HPV-related cancers. Vaccine. 2006; 24(3):S11-S25.
- Jung WW, Chun T, Sul D, Hwang KW, Kang HS, Lee DJ, et al. Strategies Against Human Papillomavirus Infection and Cervical Cancer. J Microbiol. 2004, 42:255-66
- Hernandez BU, Wickens LR, Zhu X. Transmission of HPV in heterosexual couples. Emerg Infect Dis. 2008 Jun;14(6): 888-94.
- JM M, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papilomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999, 189:12-19.
- Moberg M, Gustavsson I, Gyllensten U. Real-Time PCR Based System for Simultaneous Quantification of Human Papillomavirus Types Associated with High Risk of Cervical Cancer. J Clin Microbiol. 2003, 41:3221–28.
- Kraus I, Molden T, Holm R, Lie AK, Karlsen F, Kristensen GB, et al. Presence of E6 and E7 mRNA from Human Papillomavirus Types 16, 18, 31, 33, and 45 in the Majority of Cervical Carcinomas. J Clin Microbiol. 2006; 44:1310–17.
- Crow JM. HPV: The global burden. Nature. 2012; 488:S2–3
- Castellsagué X, de Sanjosé S, AguadoT, Louie KS, Bruni L, Muñoz J, et al. HPV and Cervical Cancer in the World 2007 Report Vaccine. 2007; 25 Suppl 3:C27-219.
- de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Muñoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. The Lancet Infectious diseases. 2007; 7:453-59.
- Serrano B, Alemany L, Tous S, Bruni L, Clifford GM, Weiss T, et al. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer. 2012; 7(1):38.
- Serrano B, Alemany L, Ruiz PA, Tous S, Lima MA, Bruni L, et al. Potential impact of a 9-valent HPV vaccine in HPV-related cervical disease in 4 emerging countries (Brazil, Mexico, India and China). Cancer Epidemiol. 2014; 38:748–56
- Nahar Q, Sultana F, Alam A, Islam JY, Rahman M, Khatun F, et al. Genital Human Papillomavirus Infection among Women in Bangladesh: Findings from a Population-Based Survey. PLoS ONE. 2014; 9(10): e107675. Available from doi:10.1371/journal.pone. 0107675
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999; 189:12-19.
- Borna NN, Tabassum S, Jahan M, Munshi SU, Nessa A. Genotyping of High Risk Human Papillomavirus (HPV) Among Cervical Precancer and Cancer Patients. Acta Medica International. Jan - Jun 2015; 2 (1): 19-28
- Khatun S, Hussain SMA, Hossain F, Choudhury A. Human papilloma virus and other risk factors of carcinoma cervix. Bangladesh Medical Journal. 2009; 38 (1): 22-27.
- Rahman T, Tabassum S, Jahan M. Risk of cervical cancer associated with HPV infection among the gynae outdoor patient. Bang Med J Khulna. 2013; 46:3-6
- Basu P, Roychowdhury S, Bafna UD, Chaudhury S, Kothari S, Sekhon R, et al. Human papillomavirus genotype distribution in cervical cancer in India: results from a multi-center study. Asian Pac J Cancer Prev. 2009; 10: 27–34.
- Senapati R, Nayak B, Kar SK, and Dwibed B. HPV Genotypes distribution in Indian women with and without cervical carcinoma: Implication for HPV vaccination program in Odisha, Eastern India. BMC Infect Dis. 2017; 17:30
- Loya A, Serrano B, Rasheed F, Tous S, Hassan M, Clavero O, et al. Human Papillomavirus Genotype Distribution in Invasive Cervical Cancer in Pakistan. Cancer. 2016; 8:72
- Srivastava S, Shahi UP, Dibya A, Gupta S, and Roy JK. Distribution of HPV Genotypes and Involvement of Risk Factors in Cervical Lesions and Invasive Cervical Cancer: A Study in an Indian Population. Int J Mol Cell Med. 2014 Spring; 3(2): 61–73.
- Mane A, Nirmalkar A, Risbud AR, Vermund SH, Mehendale SM and Sahasrabuddhe VV. HPV Genotype Distribution in Cervical Intraepithelial Neoplasia among HIV-Infected Women in Pune, India. PLoS ONE. 2012: 7(6): e38731. https://doi.org/10.1371/journal.pone.003873
- Bhatla N, Lal N, Bao YP, Ng T, Qiao YL. A meta-analysis of human papillomavirus type-distribution in women from South Asia: implications for vaccination. Vaccine. 2008; 26: 2811–17.
- Chen X, Xu H, Xu W, Zeng W, Liu J, Wu Q, et al,. [internet] Prevalence and genotype distribution of human papillomavirus in 961,029 screening tests in southeastern China (Zhejiang Province) between 2011 and 2015. 2017 Nov. Available from: www.nature.com/scientific reports.
- Baloch Z, Li Y, Yuan T, Feng Y, Liu Y, Tai W, et al. Epidemiologic characterization of human papillomavirus (HPV) infection in various regions of Yunnan Province of China. BMC Infectious Diseases.2016; 16:228
- Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003; 88: 63-73.
- Bao YP, Li N, Smith JS, Qiao YL, ACCPAB members. Human papillomavirus type distribution in women from Asia: a meta-analysis. Int J Gynecol Cancer. 2008; 18: 71-9.
- Hong D, Ye F, Chen H, LU W, Cheng Q,Hu Y,et al. Distribution of human papillomavirus genotypes in the patients with cervical carcinoma and its precursors in Zhejiang province, China. Int J Gynecol Cancer.2008;18:104-9.
- Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical intraepithelial neoplasia grade 3. Cancer Epidemiol Biomarkers Prev. 2003; 19:1675-81.
- Chaturvedi AK, Katki HA, Hildesheim A, Rodriguez AC, Quint W, Schiffman M, et al. Human papillomavirusinfection with multiple types: pattern of coinfection and risk of cervicaldisease. J Infect Dis.2011; 203:910–20
- Trottier H, Mahmud S, Costa MC, Sobrinho JP, Duarte-Franco E, Rohan TE,et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2006; 15: 1274–80
- Bruni L, Barrionuevo-Rosas L, Albero G, Serrano B, Mena M, Gómez D, et al. ICO Information Centre on HPV and Cancer (HPV Information Centre): Human Papillomavirus and Related Diseases in Bangladesh. Summary Report 27 July 2017.
- Khan S, Jaffer NN, Khan MN, Rai MA, Shafiq M, Ali A, et al. Human papillomavirus subtype 16 is common in Pakistani women with cervical carcinoma. Int J Infect Dis 2007; 11: 313–17.
- Raza SA,Franceschi S,Pallardy S, Malik FR,Avan BI, Zafar A,et al. Human papillomavirus infection in women with and without cervical cancer in Karachi, Pakistan. Br J Cancer.2010; 102: 1657–60.
- Shahsiah R, Khademalhosseini M, Mehrdad N, Ramezani F, Nadji SA. Human papillomavirus genotypes in Iranian patients with cervical cancer. Pathol Res Pract.2011; 207: 754–57
- Ghaffari SR, Sabokbar T, Mollahajian H, Dastan J, Ramezanzadeh F, Ensani F, et al. Prevalence of human papillomavirus genotypes in women with normal and abnormal cervical cytology in Iran. Asian Pac J Cancer Prev.2006;7(4):529-32.
- Alsbeih G, Ahmed R, Al-Harbi N, Venturina LA, Tulbah A, Balaraj K. Prevalence and genotypes’ distribution of human papillomavirus in invasive cervical cancer in Saudi Arabia. Gynecol Oncol. 2011; 121: 522–26.
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56.
- World Health Organization, Bangladesh: HPV vaccine introduced in Bangladesh; 2018. Available from www.searo.who.int/bangladesh/HPVvaccinelaunch/en/