Drugs are considered highly essential and sensitive items for saving lives. Pharmaceutical sector in Bangladesh has grown from strength to strength over the last 40 years and evolved from an import dependent to self-reliant, export oriented sector. But counterfeit, adulterated and sub-standard drugs, though small in percentage, still remain to be a threat in the local market. The purpose of this study is to determine potency of some essential drugs, which are used vigorously by sub-urban and rural people and manufactured by randomly selected small and medium pharmaceutical industries of Bangladesh. The study revealed that the measured potency of different essential drugs manufactured by randomly selected small pharmaceutical industries is mostly unsatisfactory. Here, it has been found that most of the drug samples did not match with the acceptance level: the potency of Amoxicillin tryhydrate capsule has been found 8.25%, 47.9%, 51.1% 19.06%, 57.06% from five different pharmaceutical industries and for Diclofenac Sodium is 44.9%, 26.8%, 27.2%, 29.9%, 19.4%, 62.12%, 15.9% and 60.76% from eight different industries. So, the Drug Administration should have a strict monitoring and controlling mechanism to the distribution of those drugs, otherwise the overall public health of the country will be at stake.
Keywords: Drug potency, Small pharmaceutical industries, Sub-standard drugs
Drugs are used to protect, cure and mitigate disease states, but these drugs may cause damage to health, and even death. While Bangladeshi pharmaceutical industries have reached the height of supplying 98% of the country′s annual drug market and increased the country′s credibility in the international markets; counterfeit, adulterated and sub-standard drugs, though small in percentage, still remain to be a threat in the local market.1-2 As reported in the media, in the absence of strict monitoring and effective control by the regulatory authority, some medium and small pharmaceutical companies are being involved repeatedly in manufacturing and marketing of counterfeit, adulterated and sub-standard drugs.
Though many factories have been already closed and license of many companies have been suspended, counterfeit, adulterated and sub-standard drugs are still reported to be extensively available mainly in the small townships and the rural areas. In the absence of proper control of the countryside, these drugs are on the market to take advantage of unconsciousness of the common people. Users of these drugs do not get the desired therapeutic effects in spite of spending their hard-earned money. Some specified drug business holders with their organized syndicates are responsible for this proliferation of counterfeit, adulterated and sub-standard drugs in the rural countryside areas.
Studies showed that some companies that are engaged in producing sub-standard drugs intentionally mimic products of some big pharmaceutical companies in fixing trade names and designing of packages and labels with a view to selling these products in the market at a cheaper rate. Sometimes these companies just supply these drugs only to the Mitford Market of Dhaka and then, from there those mimicked drugs are distributed throughout the whole country. These companies do not have their own marketing department.3-4
So, the main objective of this study is to evaluate the potency of the finished drugs that are being produced and marketed by the small and medium scale pharmaceutical industries in Bangladesh.
For potency determination of some randomly collected essential drug samples, Active Pharmaceutical Ingredients (API) of eight different drugs were collected. Analytical pure standard samples of eight different drugs were received as a kind gift samples from renowned pharmaceutical industries of Bangladesh. These were used without further purification. Name of the APIs are-Amoxicillin Trihydrate, Azithromycin, Cephradine, Cefixime Trihydrate, Ciprofloxacin Hydrochloride, Diclofenac Sodium, Metronidazole, Ranitidine Hydrochloride. For potency determination of drugs, different chemicals and solvents were used. They were- Hydrochloride acid, Methanol, Sodium Hydroxide, Monobasic Potassium phosphate, Acetoniotrile and Distilled water. Except Azithromycin, potency of other generic drug samples were analysed by UV-visible Spectrophotometry method developed by Nayon M et al, Naveed S et al, Omar SARR S et al and others and for Azithromycin, HPLC method was adopted (USP 30-NF 25).
The drug samples were collected from the rural areas which are manufactured by small and mediam pharmaceutical industries of Bangladesh. These pharmaceutical industries were enlisted in the categories of D, E and F in the 1st report of the Specialist Inspection Team that was constituted by the Parliamentary Standing Committee of Ministry of Health and Family Welfare in 2010.
For potency determination, different sample brands of each generic drug were collected from rural areas. However, the number of collected brand sample varied due to availability of sample brand. Table-1 indicates the number of brand sample that were collected for analysis. Each sample was tested three times to ensure correct potency (Table-1).
Potency determination of drug samples
For the potency determination of Cefixime trihydrate, seven samples of Cefixime trihydrate tablets of different companies were randomly collected from different areas of Bangladesh. The standard stock solution of 100μg/mL of cefixime trihydrate was prepared and was used as standard stock solution
| Name of Generic Drug Molecules | No Collected Drug Samples |
| Cefexime Trihydrate 200 mg | 7 |
| Cephradine BP 400 mg | 6 |
| Ciprofloxacin hydrochloride | 6 |
| Diclofenace Sodium 50 mg | 8 |
| Metronidazole BP 400 mg | 7 |
| Ranitidin hydrochloride 150mg | 3 |
| Amoxicillin Trihydrate | 6 |
| Azithromycin USP | 7 |
Further dilutions were made with distilled water to obtain different concentrations and the absorbance of the spectra was measured at 287 nm. The calibration curves were then constructed by plotting absorbance versus concentration and the regression equations were calculated. UV- visible spectrophotometry method developed by Nayon M et al, was used to determine the potency of these randomly collected samples.5
Twenty capsules of samples were dissembled and weight of content of each capsule was taken. The contents were grinded to make fine powder. Appropriate dilution of sample solution was prepared and absorbance was recorded. The concentration of the drug was determined from the regression equation of standard drug. After then, the potency of nine marketed products of cefixime trihydrate was calculated in percentage.
In addition, for rest of the generic drug samples except Azithromycin, the methodology followed for Cefixime trihydrate was also followed for those generic drug samples. Specifically, Naveed S et al., for Cephradine, Omar SARR S et al, for Ciprofloxacin, Pandey Get al, for Diclofenac Sodium, Das J et al, for Metronidazole, Salve P et al, for Ranitidine and Rele Ret al, for Amoxicillin were adopted respectively for potency determination of these generic drug samples.5-11
Azithromycin USP pure drug was obtained as a gift sample from one of the top Pharmaceutical industries of Bangladesh. For the potency determination of Azithromycin, seven samples of Azithromycin tablets of different companies were randomly collected from different areas of Bangladesh.
Preparation of mobile phase
Buffer:4.6 g of monobasic potassium phosphate anhydrous was transferred to a 1000 mL volumetric flask and diluted with water to volume. Then it was adjusted with 1 N sodium hydroxide to a pH of 7.5 and diluted with water to 1 L.Then mobile phase was prepared by filtering and degassing the mixture of acetonitrile and buffer. (65:35) Preparation of standard Azithromycin solution: First of all, standard azithromycin solution was prepared by taking 10 mg of USP Azithromycin RS in a 10-mL volumetric flask. Then solution of mobile phase was added up to the mark and sonicated to obtain a solution having a concentration of 1mg/mL of Azithromycin.
Preparation of sample solution: Seven market products of Azithromycin were collected. Twenty tablets from each formulation were weighed and finely powdered. An accurately weighed portion of the powder, equivalent to about 50 mg of Azithromycin was transferred to a 50-mL volumetric flask. The solution of mobile phase 25 mL was added up to the mark and sonicated for not less than 15 minutes. The solution was allowed to equilibrate to room temperature, diluted with mobile phase to volume and mixed to obtain a solution having a nominal concentration of 1 mg of Azithromycin per mL. Sample solutions of seven market products were prepared following this method. Analysis: Samples: Standard solution sand sample solution
The percentage of the labeled amount of Azithromycin (C38H72N2O12) in the portion of tablets taken: Result = (rurs)x(Cs/Cu)xPxFx100 ru= peak response of Azithromycin from the sample solution, rs= peak response of Azithromycin from the standard solution, Cs = concentration of USP Azithromycin RS in the standard solution (mg/ml), Cu = nominal concentration of Azithromycin in the sample solution (mg/ml), P = Potency of USP Azithromycin RS (μg/mg), F= conversion factor, 0.001mg/ml; Acceptance criteria: 90%-110%.
The potency of collected drug samples was determined using standard curve of respective drugs (Except Azithromycin drug samples). The potency of Azithromycin drug samples was measured using HPLC method according to USP monograph.12 The standard curves of drugs were constructed by plotting different concentration of standard solution of drug in X axis and measured absorbance at definite wavelength of respective concentration in Y axis. The regression equation was also determined from the standard curve. The standard curve with regression equation has been given in Figure-1.1-1.7
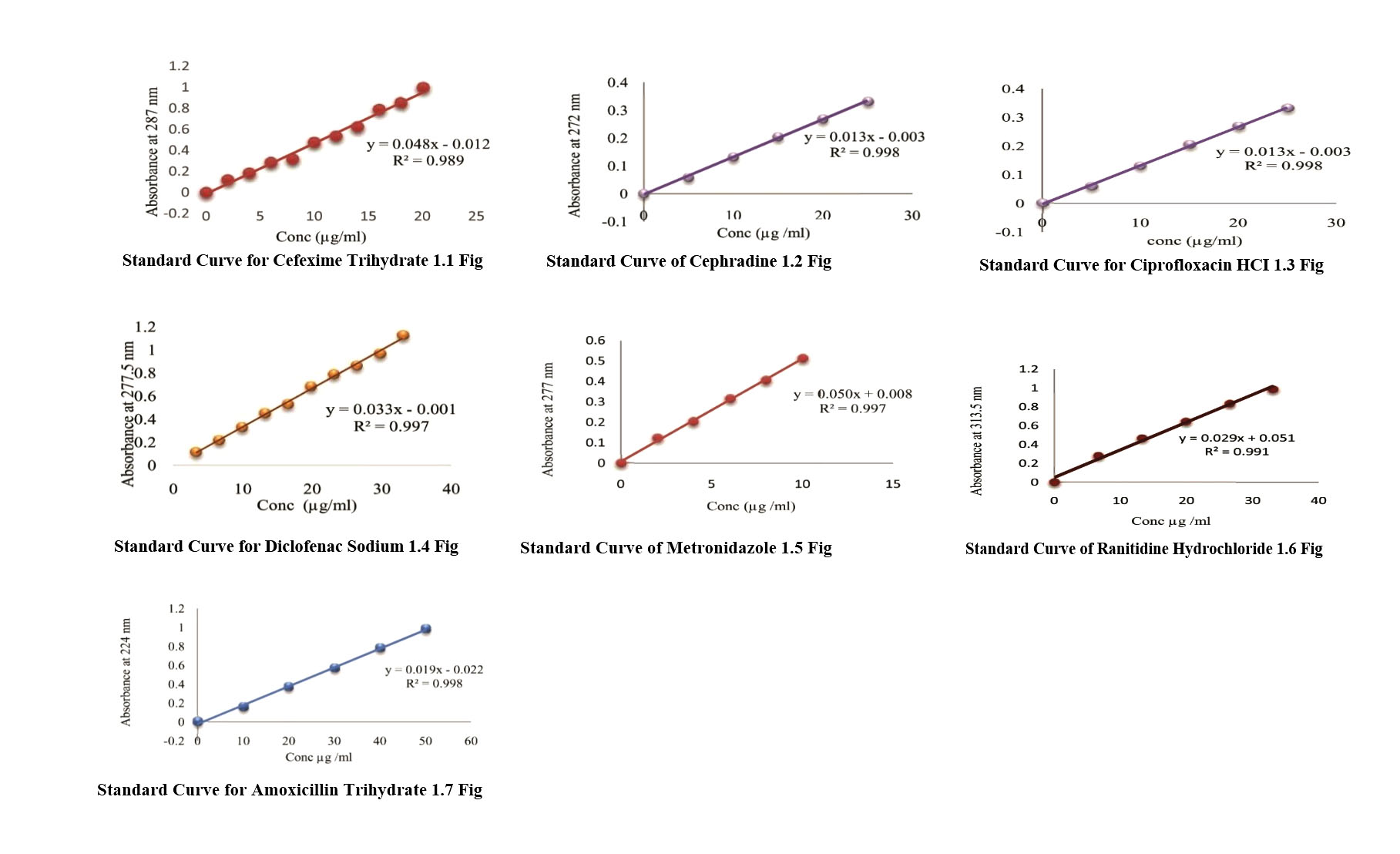
After preparing sample solution of drug samples, the absorbance value (y) of respective samples were measured at same wavelength as standard curve. Then absorbance found with unkonwn concentration were placed in the equation and the concentration (x) of the drug samplesweremeasured. The determined concentration were multiplied with the proper dilution factor and the actual amount of drug in the sample were calculated.
Amount (mg) of drug actually found in commercial samples after analysis is shown in table I and the potency of different drug samples manufactured by different small and medium pharmaceutical companies in Bangladesh shown in (Table- II).
|
Cefexime Trihydrate Capsule |
Cephradin BP Capsule |
Cipro-floxacin HCl Tablet |
Diclofenac Sodium Tablet |
Metronidazole BP Tablet |
Ranitidin HCl Tablet |
Amoxicillin Trihydrate Capsule |
Azithromycin USP Tablet |
|---|---|---|---|---|---|---|---|---|
Amount declared by the Companies |
200 mg |
400 mg |
500 mg |
50 mg |
400 mg |
150 mg |
500>mg |
500 mg |
Company A |
|
|
|
|
|
96.3 |
|
|
Company B |
129 |
|
|
22.5 |
|
|
|
|
Company C |
|
|
|
13.4 |
|
|
|
|
Company D |
|
235.6 |
352 |
13.6 |
|
|
287.5 |
|
Company E |
100.2 |
194.4 |
267 |
14.95 |
224.8 |
86.7 |
|
|
Company F |
|
|
|
|
|
|
255.5 |
|
Company G |
|
|
|
|
179.6 |
|
|
|
Company H |
|
|
|
9.7 |
59.2 |
|
|
|
Company I |
|
|
|
|
|
|
95.3 |
|
Company J |
138.28 |
303.6 |
332 |
31.06 |
274.4 |
|
376.5 |
520.7 |
Company K |
|
226.4 |
255.2 |
|
183.6 |
|
|
439 |
Company L |
|
|
|
|
|
|
|
343 |
Company M |
|
|
|
7.95 |
|
74.6 |
|
|
Company N |
142.4 |
|
|
|
|
|
|
|
Company O |
44.4 |
4.16 |
7.65 |
|
19.6 |
|
41.25 |
12.45 |
Company P |
|
|
|
|
|
|
|
474 |
Company Q |
107 |
|
|
|
|
|
|
463.5 |
Company R |
|
|
|
|
|
|
239.5 |
342.5 |
Company S |
148.5 |
242.2 |
410 |
30.4 |
255 |
|
|
|
From the above results, it has been found that most of randomly collected drugs samples of different pharmaceutical industries did not match with the acceptance level. These industries were enlisted in the categories D, E and F in the 1st Report of the Specialist Inspection Team that was constituted by the Parliamentary Standing Committee in 2010
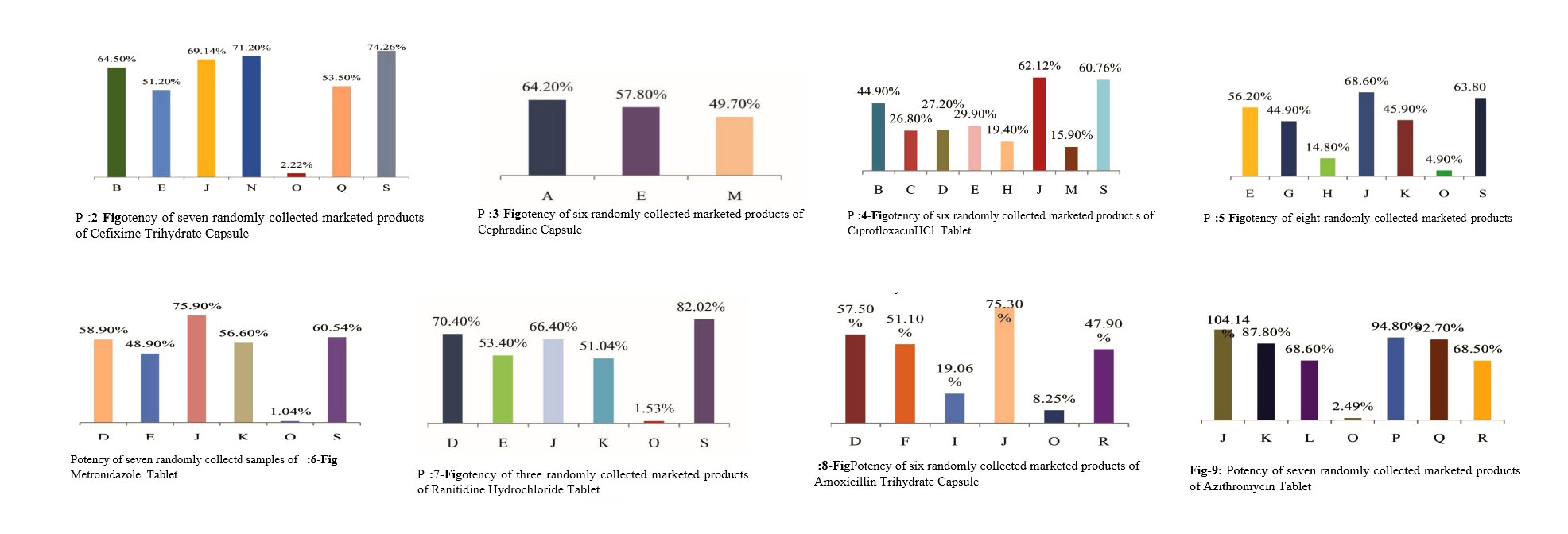
For the determination of potency of the drug samples, mainly simple UV Spectrophotometric methods were applied. According to USP monograph, each of tablet or capsule should contain not less than 90 percent and not more than 110 percent of drugs to their label claim. In this study, drug samples from eighteen pharmaceutical industries were randomly collected. Amongst these industries, a few have comparatively better potency than others. Some have moderate drug potency but still they are far from fulfilling the acceptance level. All other samples have very unsatisfactory results.(Figure 2-9)
Drug samples |
Potency (%) |
|||||||
|
Cefexime Trihydrate 200mg Capsule |
Cephradin BP 400 mg Capsule |
Cipro-floxacin HCl 500 mg Tablet |
Diclofenac Sodium 50mg Tablet |
Metronidazole BP 400mg Tablet |
Ranitidin HCl 150mg Tablet |
Amoxicillin Trihydrate 500mg Capsule |
Azithromycin USP 500mg Tablet |
CompanyA |
|
|
|
|
|
64.2 |
|
|
CompanyB |
64.5 |
|
|
44.9 |
|
|
|
|
CompanyC |
|
|
|
26.8 |
|
|
|
|
CompanyD |
|
58.9 |
70.4 |
27.2 |
|
|
57.5 |
|
CompanyE |
51.2 |
48.9 |
53.4 |
29.9 |
56.2 |
57.8 |
|
|
CompanyF |
|
|
|
|
|
|
51.1 |
|
CompanyG |
|
|
|
|
44.9 |
|
|
|
CompanyH |
|
|
|
19.4 |
14.8 |
|
|
|
CompanyI |
|
|
|
|
|
|
19.06 |
|
CompanyJ |
69.14 |
75.9 |
66.4 |
62.12 |
68.6 |
|
75.3 |
104.14 |
CompanyK |
|
56.6 |
51.04 |
|
45.9 |
|
|
87.8 |
CompanyL |
|
|
|
|
|
|
|
68.6 |
CompanyM |
|
|
|
15.9 |
|
49.7 |
|
|
CompanyN |
71.2 |
|
|
|
|
|
|
|
CompanyO |
2.22 |
1.04 |
1.53 |
|
4.9 |
|
8.25 |
2.49 |
CompanyP |
|
|
|
|
|
|
|
94.8 |
CompanyQ |
53.5 |
|
|
|
|
|
|
92.7 |
CompanyR |
|
|
|
|
|
|
47.9 |
68.5 |
CompanyS |
74.26 |
60.54 |
82.02 |
60.76 |
63.8 |
|
|
|
In addition, those collected drugs have low physical as well as packaging qualities. The finishing and coating of tablets of those drug samples were mostly questionable. The hardness of the tablets was also very low. Again, some have other physical problems like capping, lamination and sticking. Packaging design of some big companies were also copied by some of these companies.
Discussion
Drugs of these industries are extensively available in the rural and countryside areas of Bangladesh. They were not found in Dhaka city or other city areas. The study opens a hard reality that though drugs are a commodity where quality and potency cannot be compromised, some middle and small pharmaceutical industries are engaged in producing sub-standard drugs. This is an important public health threat. It is especially true in case of antibiotics. Azithromycin, Amoxicillin, Ciprofloxacin, Cephradine, Cefixime etc. are widely used antibiotics in the country because these are very effective weapons against microbial diseases prevailing in the country. But as these antibiotics are not being manufactured by maintaining proper protocol, they cannot cure these deadly diseases. Moreover, these sub-standard antibiotics are causing development of drug-resistant microorganisms that are again an additional threat both for the present and coming generations.13
To prevent this public health menace, immediate measures should be taken by the government to save our population. The industries that have been engaged in manufacturing these sub-standard drugs should be awarded strong exemplary punishment like cancellation of their manufacturing license, rigorous imprisonment, huge fine and confiscation of their properties.
Moreover, the study also focus that these industries are marketing their sub-standard drugs in the remote rural areas where monitoring by the Drug Administration is feeble or absent. Taking this opportunity, these companies in collaboration with the retail drug stores are selling their sub-standard products. Thus, it is urged that as a regulatory body, the Drug Administration should improve their monitoring activities effectively so that no sub-standard drug can infiltrate the market and cause damage to public health. One of the reasons as to why some middle and small pharmaceutical industries have been engaging themselves to produce sub-standard drugs can be that they do not have the necessary manufacturing facilities to produce standard quality drugs. In this case, before issuing a new or renewing an existing manufacturing license authority must ensure that all required facilities are being present in the premise of the industry.
Conclusion
In this study, potency of some randomly selected essential drug samples of some medium and small pharmaceutical industries of Bangladesh were analyzed and found unsatisfactory. As drugs are for the well being of health and health can’t be risked, thus proper regulatory control and monitoring should be applied for the distribution of these sub-standard drugs manufactured by the medium and small pharmaceutical industries of Bangladesh. As an outcome of this work, it feels necessary to make the following recommendations:
Based on the findings of the study, it is recommended that the Drug Administration should come out of its monitoring limitations, and introduce a strengthened and transparent monitoring mechanism covering the whole chain across the country; manufacturing licenses should not be renewed or issued to drug companies which are non compliant with the WHO′s GMP Guidelines of 1975 and standards set out under the laws and regulations of the country; and licenses of such companies may be cancelled or suspended until the satisfactory correction. Furthermore, for small and medium pharmaceutical companies, the Drug Administration should stop issuing product registration of high technology drugs or drugs that need dedicated areas and machines to manufacture such as antibiotics. These types of product registrations already issued to these companies may be withdrawn or suspended immediately.
References
- Tazin F. Pharmaceutical Industry of Bangladesh: Progress and Prospects. The Millennium University Journal. 2016;1:19-20.
- Kabir K. Bangladesh Pharma Industry: Opportunities in Global Generics [Internet]. 2016 p. 4-5. Available at www.jetro.go.jp /ext_images/world/asia/bd/seminar_reports/20160413/p4.pdf
- Rabbi, R. (2015). The Harmful Effects of Adulterated Medicine. The Daily Jugantor, [online] p.1. Available at:www.jugantor.com /old/first-page/2015/08/04/302467 [Accessed 15 Jul. 2016].
- Mahmud S. Did not Stop Adulterated and Substandard Drugs. Bangladesh Protidin [Internet]. 2015 [cited 5 June 2016];:12. Available at: http://www.ebdpratidin.com/arc /pre_page /2015-11-24/12
- Nayon AU, Nesa J, UddinMN, Amran MS, Bushra U.Development and validation of UV Spectrometric Method for the Determination of Cefixime trihydrate in Bulk and Pharmaceutical Formulation. 2013;301-05.
- Naveed S, Jaweed L. UV Spectrophotometric Assay of Different Brands of Cephradine. Health Sciences Research. 2014; 1:84-7.
- Sarr SO, Fall D, Ndiaye SM, Diedhiou A, Diop A, Ndiaye B, Diop YM. Development and validation of a simple and economical spectrofluorimetric method for estimation of quinine in pharmaceutical dosage forms. Int J of Biol and Chem Sci. 2013; 7:366-76.
- Pandey G. Spectrophotometric methods for estimation of diclofenac sodium in tablets. Int J of Biomedand Adv Res. 2013; 4:77-82.
- Das J, Dhua M. UV-spectrophotometric assay method development and validation of metronidazole in bulk and tablet formulation. J of Pharm Sci Tech. 2014; 3:109.
- Salve P, Gharge D, Kirtawade R, Dhabale P, Burade K. Simple validated spectroscopic method for estimation of Ranitidine from tablet formulation. Int J Pharm Tech Res. 2010; 2:2071-4.
- Rele R.Simultaneous UV-Spectrophotometric estimation of amoxicillin and carbocisteine by area under curve method in combined dosage form. 2015;5:1-7.
- United States Pharmacopeia and National Formulary (USP 30-NF 25).Rockville, MD: United States Pharmacopeial Convention; 2007.
- Knobler S. The resistance phenomenon in microbes and infectious disease vectors. Washington, D.C.: National Academies Press; 2003.