Principal Contact
Abstract:
Infantile hemangiomas are the most common vascular tumor of infancy and childhood. Sometimes these lesions interfere with normal function & produce serious disfigurement that is unlikely to resolve on its own and then treatment is required. While evidence most supports the use of corticosteroids, there is no well- studied or Federal Drug Administration (FDA) approved systemic therapy for haemangiomas of Infancy. Dramatic improvement of complicated haemangioma by propranolol has recently been reported, but to date, details for initiating therapy, monitoring and potential risks in relation to Corticosteroids were not compared in a large scale. This research was designed to observe the effectiveness of oral propranolol compared to oral corticosteroid aimed at treatment of clinically important groups of infantile hemangiomas that require aggressive treatment. We conducted a randomized control study among the diagnosed case of infantile hemangiomas, age of ≤10 years. The total sample size was 60 (30 for propranolol group i.e. in group A, and 30 for corticosteroid group i.e. in group B) & grouping was done with the simple random technique. A clinical & photograph based VAS (Visual Analogue Scale) scale with a defined monitoring schedule was used for evaluation of treatment response. Our study result showed, (3.33%) patients of group A, response to color change (red-purple-grey) within 1st month of treatment whereas none of the patient (0.00%) of group B had any response to color by this time. Patients response to propranolol therapy was also continued even up to 5th month (3.33%) but it was absent (0.00%) in corticosteroid therapy even after 4th month & p= 0.025, that was statistically significant. Regarding the mean size (diameter) of the tumor, most of the tumor size reduced and near to stabilize at 4th month in group A, but in group B, the rate was slower and needed longer time (5-6 month), the p value was 0.030. Again 60% of tumor became non-palpable at 3rd week of treatment in group A patients, but in group B, 70% of tumor was still remain palpable on that time & p=0.001, that was statistically significant. Again, in Propranolol therapy group, the rate of complications was (24%) whereas in Corticosteroid therapy group, it was (76%).The p value was 0.020 that was also statistically significant. Hence, the present study results denote that, oral propranolol can be considered as an emerging and effective treatment over oral corticosteroid therapy for infantile hemangiomas.
Key word: Propranolol, Corticosteroids, Infantile haemangiomas
Key word: Propranolol, Corticosteroids, Infantile haemangiomas
Introduction:
Infantile hemangioma (IH) comprises majority of the vascular anomalies, may be associated with significant morbidity. Approximately, 20% of hemangiomas result in pain, bleeding, ulceration, infection, or functional impairment with vision,feeding, or breathing necessitating medical or surgical treatment.1,2 Larger and /or multiple cutaneous IHs may be associated with high-output cardiac failure, cosmetic disfigurement and psychological morbidity in both child and the family. Nevertheless, some hemangiomas can impair vital functions or cause morbidity and mortality.3 Hemangiomas are regarded as problematic hemangiomas when they have massive growth, bleeding, ulceration, cause disfigurement or impair normal function or cosmetic development. Complications rate and need for treatment varied according to location of hemangiomas. Common locations for problematic haemangioma include face, ear, orbit, and airway and anogenital region. These haemangiomas subsequently require early and aggressive treatment for ideal functional and cosmetic outcomes.4 Currently no medications exist that are specially labeled to treat IHs.5 The current treatment options for complicated haemangiomas include the use of systemic or intralesional corticosteroids, chemotherapeutic agents (vincristine, alpha interferon), laser therapy, surgical resection, or a combination of these treatments.6 However, all these options have potential side effects or unknown long-term safety. Propranolol hydrochloride (a nonselective β-blocker) has recently been introduced as a novel pharmacologic agent for the treatment of infantile hemangiomas. In different case series, it has been observed that, propranolol hydrochloride produce dramatic involution of IHs.1,7
Till date, as there are no well studied or federal drugs administraton (FDA) approved gold standard systemic treatments for infantile hemangiomas and existing treatment modalities have many adverse effects. Hence, treatment of infantile hemangioma specially complicated one is challenging.5 The aim of the current study is to explore a comparative impact between oral corticosteroids (that is till now use as first line therapy) and oral propranolol therapy (that is recently considered first choice of drug) in the treatment of infantile hemangioma regarding their safety and effectiveness in pediatric population.
Throughout the world very few studies are carried out about efficacy of propanolol in comparison to systemic corticosteroids. In Bangladesh the number is very little and till now, here so far no well documented large scale study has been done. But like many physicians throughout the world, propranolol has also made a new hope among the physicians of Bangladesh. Hence, before adoption of a drug, it is important to evaluate different approaches and provide clinical study on its effectiveness in perspective of Bangladeshi population. The key objective of the study is to determine whether oral Propranolol therapy is safe, effective and superior to systemic corticosteroids for treating infantile hemagiomas.
Infantile hemangioma (IH) comprises majority of the vascular anomalies, may be associated with significant morbidity. Approximately, 20% of hemangiomas result in pain, bleeding, ulceration, infection, or functional impairment with vision,feeding, or breathing necessitating medical or surgical treatment.1,2 Larger and /or multiple cutaneous IHs may be associated with high-output cardiac failure, cosmetic disfigurement and psychological morbidity in both child and the family. Nevertheless, some hemangiomas can impair vital functions or cause morbidity and mortality.3 Hemangiomas are regarded as problematic hemangiomas when they have massive growth, bleeding, ulceration, cause disfigurement or impair normal function or cosmetic development. Complications rate and need for treatment varied according to location of hemangiomas. Common locations for problematic haemangioma include face, ear, orbit, and airway and anogenital region. These haemangiomas subsequently require early and aggressive treatment for ideal functional and cosmetic outcomes.4 Currently no medications exist that are specially labeled to treat IHs.5 The current treatment options for complicated haemangiomas include the use of systemic or intralesional corticosteroids, chemotherapeutic agents (vincristine, alpha interferon), laser therapy, surgical resection, or a combination of these treatments.6 However, all these options have potential side effects or unknown long-term safety. Propranolol hydrochloride (a nonselective β-blocker) has recently been introduced as a novel pharmacologic agent for the treatment of infantile hemangiomas. In different case series, it has been observed that, propranolol hydrochloride produce dramatic involution of IHs.1,7
Till date, as there are no well studied or federal drugs administraton (FDA) approved gold standard systemic treatments for infantile hemangiomas and existing treatment modalities have many adverse effects. Hence, treatment of infantile hemangioma specially complicated one is challenging.5 The aim of the current study is to explore a comparative impact between oral corticosteroids (that is till now use as first line therapy) and oral propranolol therapy (that is recently considered first choice of drug) in the treatment of infantile hemangioma regarding their safety and effectiveness in pediatric population.
Throughout the world very few studies are carried out about efficacy of propanolol in comparison to systemic corticosteroids. In Bangladesh the number is very little and till now, here so far no well documented large scale study has been done. But like many physicians throughout the world, propranolol has also made a new hope among the physicians of Bangladesh. Hence, before adoption of a drug, it is important to evaluate different approaches and provide clinical study on its effectiveness in perspective of Bangladeshi population. The key objective of the study is to determine whether oral Propranolol therapy is safe, effective and superior to systemic corticosteroids for treating infantile hemagiomas.
Materials and Methods:
The randomised controlled trial was carried out during the period of January 2013 to June 2013 in the Department of Surgery, Mymensingh Medical College Hospital, Mymensingh among the children of 1 day to 10 years of age, who were diagnosed as haemangiomas. The admission register of the Mymensingh Medical College Hospital was used as the sample frame. Registered patients with Haemangiomas symptoms were identified for follow-up. Detail explanation to all parents or guardians about the purpose and procedure of study and advantages and disadvantages of both groups of therapy. The Participants' freedom regarding the refusal of study was kept and if they agree then random selection of the study group was done. Informed written consent (Both English and Bangla version) was obtained for each patient. Assurance was given regarding all sorts of confidentiality. Any economic burden to the parents will be a basic consideration. The study was approved by the BMRC Ethical Committee when it was submitted for support.
After explanation to the parents about the study, they were requested to draw one of the slip from the box blindly which were kept in outpatient department. Grouping of the patients for propranolol therapy (Group-A) and those for corticosteroid therapy (Group-B) was thus made by simple random sampling by means of lottery with sample size 60 (30 in each group). They were followed- up on regular basis and the observations were recorded.
The prevalence rate for infantile haemangioma was considered as 0.1% according the study of Indian subcontinent8 because no data were available regarding prevalence rate of infantile hemangiomas among Bangladeshi population. Data were collected using a structured questionnaire from selected population groups of outpatient department of pediatric surgery. It was based on serial photographic evaluation and clinical examination findings. Variables studied were of age group, total duration of treatment, rate and pattern of complications and rate of drug response. Proliferative haemangioma, problema-tic haemangiomas comprising-imminent, undesirable functional or cosmetic outcomes were included and patients having any cardiac pathology, significant lungs pathology or patients with congenital diabetes mellitus or hypoglyacemia, gastro-esophageal reflux disorder, any internal, visceral or calcified hemangiomas and parents/patients who disagreed to particpate , were excluded.
Till date, there is so far no documented and established scale for measurement of therapeutic response of hemangioma. Several authorsused VAS (Visual Analogue Scale) in their studies.5,9,10 On the basis of these studies, in this present research, researchers used a clinical and photograph based VAS scale as evaluation grading schedule of hemangioma where both front and lateral pictures of every lesion were taken before and after treatment in every visit. The three clinical observers of the study scored the severity of the IHs grading from (X- 0), considered X (10) as the original IH that is rapidly proliferating hemangioma before treatment and X (0) as completely normal skin after treatment. The mean of the three independent measurements was used as the valid scoring value. Measurements were obtained through photographs taken during the scheduled visits. Although the final outcome of ulceration was recorded, the extent or progress of ulceration was not considered for the visual scale severity scoring. According to VAS, patient's response to applied treatment for IH's was categorized as six to one, considering six (Grade 0-I) as the excellent, five (Grade II-III) as very good, four (Grade IV-V) as good/fair, three (Grade VI-VII) as poor, two (VIII-IX) as very poor and one (X) as the non responsive.
An oral dose of 2 mg/kg/day divided three times daily was deemed safe and potentially effective as determined by one study.10 In this study, it also administered both oral propanolol and oral corticosteroid tablets as 2mg/kg/day in divided dose in each patient of respective groups in full stomach, and dose adjustments was done after patient assessments on each hospital visits (total 10 visits in 6 months) according to monitoring schedule. Before that, in brief, careful patient history and physical examination were performed. As baseline investigations, electrocardiogram, echocardiogram, complete blood count, random blood sugar and X-ray chest were performed and consultation with paediatric cardiologist was done for all study population. Partial and no responders to propranolol underwent adjuvant therapy with steroid injection, or surgical excision. The data were analyzed by using SPSS version 17.0 and all analytical tests level of significant was set at 5% and p<0.05 was considered significant.
The randomised controlled trial was carried out during the period of January 2013 to June 2013 in the Department of Surgery, Mymensingh Medical College Hospital, Mymensingh among the children of 1 day to 10 years of age, who were diagnosed as haemangiomas. The admission register of the Mymensingh Medical College Hospital was used as the sample frame. Registered patients with Haemangiomas symptoms were identified for follow-up. Detail explanation to all parents or guardians about the purpose and procedure of study and advantages and disadvantages of both groups of therapy. The Participants' freedom regarding the refusal of study was kept and if they agree then random selection of the study group was done. Informed written consent (Both English and Bangla version) was obtained for each patient. Assurance was given regarding all sorts of confidentiality. Any economic burden to the parents will be a basic consideration. The study was approved by the BMRC Ethical Committee when it was submitted for support.
After explanation to the parents about the study, they were requested to draw one of the slip from the box blindly which were kept in outpatient department. Grouping of the patients for propranolol therapy (Group-A) and those for corticosteroid therapy (Group-B) was thus made by simple random sampling by means of lottery with sample size 60 (30 in each group). They were followed- up on regular basis and the observations were recorded.
The prevalence rate for infantile haemangioma was considered as 0.1% according the study of Indian subcontinent8 because no data were available regarding prevalence rate of infantile hemangiomas among Bangladeshi population. Data were collected using a structured questionnaire from selected population groups of outpatient department of pediatric surgery. It was based on serial photographic evaluation and clinical examination findings. Variables studied were of age group, total duration of treatment, rate and pattern of complications and rate of drug response. Proliferative haemangioma, problema-tic haemangiomas comprising-imminent, undesirable functional or cosmetic outcomes were included and patients having any cardiac pathology, significant lungs pathology or patients with congenital diabetes mellitus or hypoglyacemia, gastro-esophageal reflux disorder, any internal, visceral or calcified hemangiomas and parents/patients who disagreed to particpate , were excluded.
Till date, there is so far no documented and established scale for measurement of therapeutic response of hemangioma. Several authorsused VAS (Visual Analogue Scale) in their studies.5,9,10 On the basis of these studies, in this present research, researchers used a clinical and photograph based VAS scale as evaluation grading schedule of hemangioma where both front and lateral pictures of every lesion were taken before and after treatment in every visit. The three clinical observers of the study scored the severity of the IHs grading from (X- 0), considered X (10) as the original IH that is rapidly proliferating hemangioma before treatment and X (0) as completely normal skin after treatment. The mean of the three independent measurements was used as the valid scoring value. Measurements were obtained through photographs taken during the scheduled visits. Although the final outcome of ulceration was recorded, the extent or progress of ulceration was not considered for the visual scale severity scoring. According to VAS, patient's response to applied treatment for IH's was categorized as six to one, considering six (Grade 0-I) as the excellent, five (Grade II-III) as very good, four (Grade IV-V) as good/fair, three (Grade VI-VII) as poor, two (VIII-IX) as very poor and one (X) as the non responsive.
An oral dose of 2 mg/kg/day divided three times daily was deemed safe and potentially effective as determined by one study.10 In this study, it also administered both oral propanolol and oral corticosteroid tablets as 2mg/kg/day in divided dose in each patient of respective groups in full stomach, and dose adjustments was done after patient assessments on each hospital visits (total 10 visits in 6 months) according to monitoring schedule. Before that, in brief, careful patient history and physical examination were performed. As baseline investigations, electrocardiogram, echocardiogram, complete blood count, random blood sugar and X-ray chest were performed and consultation with paediatric cardiologist was done for all study population. Partial and no responders to propranolol underwent adjuvant therapy with steroid injection, or surgical excision. The data were analyzed by using SPSS version 17.0 and all analytical tests level of significant was set at 5% and p<0.05 was considered significant.
Result:
A total of sixty patients having infantile haemangiomas (IH) trialed under this study, 53% of children under Propranolol therapy were of 0-6 month age, against 50% of children of the same age group under Corticosteroid therapy. The corresponding patients were 40% and 43% respectively from age group 7-12 month. An equal number (7%) of patients in both groups was of more than one year of age. Distribution of patients by age groups is shown in figure 1.
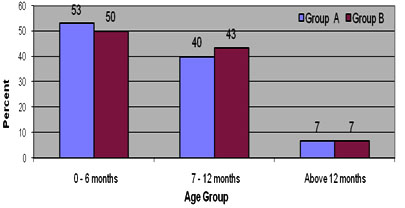
Fig 1: Distribution of patients by age groups
It was observed that in group A, patients response to color change due to propranolol therapy (3.33%) had occurred in 1st month of treatment whereas there was no patient (0.00%) to response to color change by corticosteroid therapy within 1st month. Again, though in most of the cases attainment of normalcy (near normal or almost normal colour) appeared in 3rd month of treatment in both groups (13.33% in group A and 10.00% in group B accordingly) but patients response to propranolol therapy was also continued even up to 5th month (3.33%) and that was absent (0.00%) in corticosteroid therapy after 4th month. It was also observed that there was none in both groups to show any response to color after 5th month. Statistical analysis (t-test) of the findings of two groups showed, p= 0.025, which is<0.05, that is statistically significant. So, it could be inferred that, patients with Propranolol therapy had an early, quicker as well as sustain response than that of Corticosteroid therapy to return the normalcy of skin color. The result of distribution of patients by Colors is shown in figure 2.
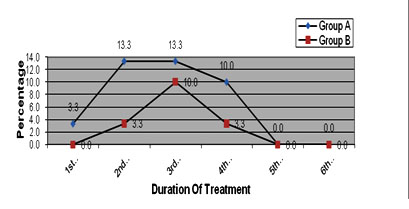
Fig. 2. Distribution of patients attained normalcy in color by duration of treatment
It was noted that in group A, at 4th month of treatment, most of the tumor size had been reduced and stabilized, whereas in group B, though tumor size had been reduced by duration of treatment, but as compared to group A patients the rate was slower and needed longer time. So, it can be inferred that, propranolol plays more effective role to reduce the tumor volume than corticosteroid (p=0.030). The result is shown in table-I.
On comparison of softness and palpability of the tumor among the two groups of patients it was noted that at 3rd week of treatment in group A, about 60% of tumor became non-palpable & soft, but in group B, 70% of tumor was remain palpable. Again, when at 6th month, 97% of tumor became non palpable while 73% tumor of group B was non palpable. Statistical analysis (t-test) of the study findings of two groups (Group A and Group B). So statistically the results was significant and it indicates that propranolol had more rapid action to make the lesions soften & non palpable over corticosteroid (p=0.001). The result is shown in figure III.
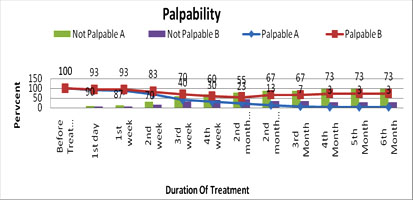
Fig. 3: Percent distribution of patients by Palpability on duration of intervention
In present study, the rate of complications was less in patients with Propranolol therapy (24%) than those with Corticosteroid therapy (76%). Among the complications, the most common one in Group A was anorexia 4 (12%) & in Group B it was Cushingoid facies 10 (35%). The other complications of group A like increased sleepiness and allergic rash were observed only two(2) patients respectively and relatively a more number of patients in group B was noted with various complications such as hypertension 3 (11%), irritability 6 (18%) & gastro intestinal upset 4(12%) table II.
It was observed that the recipients of the highest grades 6 (33%) and 5 (30%) were from group-A against corresponding 17% recipients in group-B. The highest number of recipients in Group B was grade 4 (40%). The t- test of the results of two groups showed, p= 0.020, which is <0.05, that is statistically significant. It indicated that response to Propranolol therapy is much more positive than that of corticosteroid therapy (figure 4).
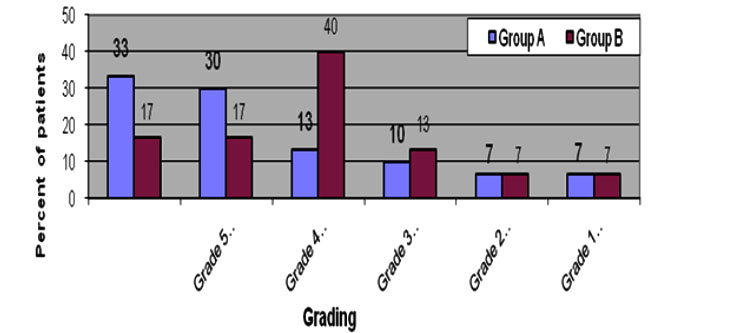
Figure 4: Distribution of patients obtaining drug response scores
In this study, the mean age group of the study population was of 6.9 months and most of the patients in both groups presented at less than one (<1) year of age. This is consistent with the typical presentation period of haemnagioma and most of the time that appear after seven days of birth.12
About patients response to color change, (3.33%) patients in study group a (propranolol therapy) showed, red to purple to grey in color within first month of treatment whereas none of the patient of group B (corticosteroid therapy) had any response to color on that time. Though in most of the cases attainment of normalcy (near normal or almost normal color) appeared in third month of treatment in both groups (13.33% in group A and 10.00% in group B accordingly). Response of patients to propranolol therapy was continued even up to fifth month (3.33%) and that was in corticosteroid therapy after fourth month (p= 0.025). So, it could be inferred that, patients with propranolol therapy had an early, quicker as well as longer response than that of corticosteroid therapy. This findings correlate with the study findings of the average response score of 2.38, 2.40, 2.69 and 3.51 after 1 month, 2 months, 3 to 4 months and >5 months of propranolol therapy.1
Regarding mean size (diameter) of the tumor, in group A, most of the tumor size reduced and near to stabilize at 4th month of treatment (2.77 mm at fourth month), whereas in group B, though tumor size had been reduced by that period (6.22 mm, at fourth month), but as compared to group A, the rate was slower and needed longer time (5-6 month) (p=0.030). This result was found a little bit defer from a study, where regression rate of the mean dimension of the lesion was 38%± 15 (range 15-50%, median 45%) at the second month of propranolol therapy.3 But, as it was an ongoing study result so, the appropriate result might not be reflected.
In this study, an abrupt change had been occurred at third week of treatment in group A, where 60% of tumor became non-palpable but in group B, 70% of tumor remained palpable at that time (p=0.001), The study could be correlated with the study of San V et al 2009 in the point that, propranolol played more active role and less time than corticosteroid to make the tumor soften and non-palpable.13 Almost similar result was observed in a study where clinical evidence of regression of tumors by propranolol therapy was seen within 30 days in all 36 cases and completely regressed within 7 months and mean duration of treatment was 4.1 months.14
In the current study, the rate of complications was less in patients with propranolol therapy (24%) than those with corticosteroid therapy (76%). Among the complications, the most common one in Group A was anorexia 4 (12%) and in Group B it was cushingoid facies 10 (35%). The other complications like increase sleepiness and allergic rash were observed only two (2) patients respectively in group A, and relatively a more number of patients in group B was noted with various complications such as hypertension 3 (11%), irritability 6 (18%) and Gastro intestinal upset 4 (12%). These complications usually found to appear after 3rd or 4th dose of intervention in each group. However, they were managed accordingly. Other researcher found that, patients who were treated with propranolol therapy, had suffered with somnolence (27.3%) more than the other complications such as gastroesophageal reflux 2 (9.1%), allergic rash 1 (4.5%), and respiratory syncytial virus exacerbation1 (4.5%) and in another study it was noticed that patients who received propranolol therapy,1(1.47%) had developed hypoglycemia and 2(2.94%) patients were suffered with non-specific skin eruption.1,3 In another study, it was noted that, a significant number of children (61%) developed puffiness of the face (cushingoid facies) after 4-6 weeks of high-dose oral prednisolone , thirty seven children (8%) developed oral thrush and twenty three (5%) suffered from loose motion. Fungal infection of the skin occurred in 16 infants (3.46%) and frank abscess developed in seven (1.5%). Two infants showed retarded growth and another developed pneumonia during the course of the treatment.15 So, comparing the different researches with current one, the complications in propranolol therapy though varies from patient to patient but were relatively less common and not a great trouble of tolerance and to manage.
Regarding drug response, the current study results show that, in groups A, excellent responder was of (33%) and they were graded as six, but, in group B only (17 %) of patients had achieved that score. The highest numbers (40%) of respondent in Group B were in category of good responder and their grade was in 4. The mean percent of poor responder (grade 3) in two groups were comparable (10 % ,13% group A and B respectively) , but the mean percent of very poor responder (grade 2) and non responder (grade1) were same (7%) in both groups (p=0.020). This result was also more or less supported by a study where respondents to propranolol were graded as excellent (n= 16.50%), partial responder (n=15.47%) & non responder (n=11.3%).1
Conclusion
In respect to the derived study findings it has been suggested that propranolol therapy could be considered as an emerging and effective treatment over oral corticosteroid therapy for infantile hemangiomas regarding patients' improvement and safety. As the study was carried out only in a single tertiary care hospital of the country with a limited sample size and within a short duration it can be considered as 1st line of treatment replacing the current one (oral corticosteroid). However, a multi centred study with a larger sample size and longer duration is recommended.
A total of sixty patients having infantile haemangiomas (IH) trialed under this study, 53% of children under Propranolol therapy were of 0-6 month age, against 50% of children of the same age group under Corticosteroid therapy. The corresponding patients were 40% and 43% respectively from age group 7-12 month. An equal number (7%) of patients in both groups was of more than one year of age. Distribution of patients by age groups is shown in figure 1.

It was observed that in group A, patients response to color change due to propranolol therapy (3.33%) had occurred in 1st month of treatment whereas there was no patient (0.00%) to response to color change by corticosteroid therapy within 1st month. Again, though in most of the cases attainment of normalcy (near normal or almost normal colour) appeared in 3rd month of treatment in both groups (13.33% in group A and 10.00% in group B accordingly) but patients response to propranolol therapy was also continued even up to 5th month (3.33%) and that was absent (0.00%) in corticosteroid therapy after 4th month. It was also observed that there was none in both groups to show any response to color after 5th month. Statistical analysis (t-test) of the findings of two groups showed, p= 0.025, which is<0.05, that is statistically significant. So, it could be inferred that, patients with Propranolol therapy had an early, quicker as well as sustain response than that of Corticosteroid therapy to return the normalcy of skin color. The result of distribution of patients by Colors is shown in figure 2.

It was noted that in group A, at 4th month of treatment, most of the tumor size had been reduced and stabilized, whereas in group B, though tumor size had been reduced by duration of treatment, but as compared to group A patients the rate was slower and needed longer time. So, it can be inferred that, propranolol plays more effective role to reduce the tumor volume than corticosteroid (p=0.030). The result is shown in table-I.
Table I: Comparison of observed changes in mean size( in diameters) of tumor by duration of treatment:
Duration of treatment |
Group A (n=30) |
Group B (n=30) |
|---|---|---|
Before treatment |
9.956 |
9.682 |
1st day of treatment |
9.930 |
9.626 |
1st week of treatment |
8.287 |
8.979 |
2nd week of treatment |
6.271 |
8.183 |
3rd week of treatment |
4.993 |
8.741 |
4th week of treatment |
4.555 |
7.582 |
1st 15 days of 2nd month |
3.490 |
7.520 |
Last 15 days of 2nd month |
3.676 |
7.000 |
3rd month of treatment |
3.546 |
6.880 |
4th month of treatment |
2.777 |
6.225 |
5th month of treatment |
2.300 |
5.536 |
6th month of treatment |
2.300 |
5.536 |
On comparison of softness and palpability of the tumor among the two groups of patients it was noted that at 3rd week of treatment in group A, about 60% of tumor became non-palpable & soft, but in group B, 70% of tumor was remain palpable. Again, when at 6th month, 97% of tumor became non palpable while 73% tumor of group B was non palpable. Statistical analysis (t-test) of the study findings of two groups (Group A and Group B). So statistically the results was significant and it indicates that propranolol had more rapid action to make the lesions soften & non palpable over corticosteroid (p=0.001). The result is shown in figure III.

In present study, the rate of complications was less in patients with Propranolol therapy (24%) than those with Corticosteroid therapy (76%). Among the complications, the most common one in Group A was anorexia 4 (12%) & in Group B it was Cushingoid facies 10 (35%). The other complications of group A like increased sleepiness and allergic rash were observed only two(2) patients respectively and relatively a more number of patients in group B was noted with various complications such as hypertension 3 (11%), irritability 6 (18%) & gastro intestinal upset 4(12%) table II.
Table II: Percent distribution of patients by complications after interventions
Observed complications |
Group A |
Group B |
|---|---|---|
Anorexia |
4 (12) |
- |
Increase sleepiness |
2 (6) |
- |
Allergic rash |
2 (6) |
- |
Hypertension |
- |
3 (11) |
CushingoidFaces |
- |
10 (35) |
Irritability |
- |
6 (18) |
Gastro intestinal upset |
- |
4 (12) |
It was observed that the recipients of the highest grades 6 (33%) and 5 (30%) were from group-A against corresponding 17% recipients in group-B. The highest number of recipients in Group B was grade 4 (40%). The t- test of the results of two groups showed, p= 0.020, which is <0.05, that is statistically significant. It indicated that response to Propranolol therapy is much more positive than that of corticosteroid therapy (figure 4).

Discussion
Infantile hemangiomas (IH) are benign vascular neoplasm and usually do not necessitate intervention. However, those are deforming, destructive or obstructing can cause numerous functional or cosmetic problems and requires pharmacotherapy or resection.11,16In this study, the mean age group of the study population was of 6.9 months and most of the patients in both groups presented at less than one (<1) year of age. This is consistent with the typical presentation period of haemnagioma and most of the time that appear after seven days of birth.12
About patients response to color change, (3.33%) patients in study group a (propranolol therapy) showed, red to purple to grey in color within first month of treatment whereas none of the patient of group B (corticosteroid therapy) had any response to color on that time. Though in most of the cases attainment of normalcy (near normal or almost normal color) appeared in third month of treatment in both groups (13.33% in group A and 10.00% in group B accordingly). Response of patients to propranolol therapy was continued even up to fifth month (3.33%) and that was in corticosteroid therapy after fourth month (p= 0.025). So, it could be inferred that, patients with propranolol therapy had an early, quicker as well as longer response than that of corticosteroid therapy. This findings correlate with the study findings of the average response score of 2.38, 2.40, 2.69 and 3.51 after 1 month, 2 months, 3 to 4 months and >5 months of propranolol therapy.1
Regarding mean size (diameter) of the tumor, in group A, most of the tumor size reduced and near to stabilize at 4th month of treatment (2.77 mm at fourth month), whereas in group B, though tumor size had been reduced by that period (6.22 mm, at fourth month), but as compared to group A, the rate was slower and needed longer time (5-6 month) (p=0.030). This result was found a little bit defer from a study, where regression rate of the mean dimension of the lesion was 38%± 15 (range 15-50%, median 45%) at the second month of propranolol therapy.3 But, as it was an ongoing study result so, the appropriate result might not be reflected.
In this study, an abrupt change had been occurred at third week of treatment in group A, where 60% of tumor became non-palpable but in group B, 70% of tumor remained palpable at that time (p=0.001), The study could be correlated with the study of San V et al 2009 in the point that, propranolol played more active role and less time than corticosteroid to make the tumor soften and non-palpable.13 Almost similar result was observed in a study where clinical evidence of regression of tumors by propranolol therapy was seen within 30 days in all 36 cases and completely regressed within 7 months and mean duration of treatment was 4.1 months.14
In the current study, the rate of complications was less in patients with propranolol therapy (24%) than those with corticosteroid therapy (76%). Among the complications, the most common one in Group A was anorexia 4 (12%) and in Group B it was cushingoid facies 10 (35%). The other complications like increase sleepiness and allergic rash were observed only two (2) patients respectively in group A, and relatively a more number of patients in group B was noted with various complications such as hypertension 3 (11%), irritability 6 (18%) and Gastro intestinal upset 4 (12%). These complications usually found to appear after 3rd or 4th dose of intervention in each group. However, they were managed accordingly. Other researcher found that, patients who were treated with propranolol therapy, had suffered with somnolence (27.3%) more than the other complications such as gastroesophageal reflux 2 (9.1%), allergic rash 1 (4.5%), and respiratory syncytial virus exacerbation1 (4.5%) and in another study it was noticed that patients who received propranolol therapy,1(1.47%) had developed hypoglycemia and 2(2.94%) patients were suffered with non-specific skin eruption.1,3 In another study, it was noted that, a significant number of children (61%) developed puffiness of the face (cushingoid facies) after 4-6 weeks of high-dose oral prednisolone , thirty seven children (8%) developed oral thrush and twenty three (5%) suffered from loose motion. Fungal infection of the skin occurred in 16 infants (3.46%) and frank abscess developed in seven (1.5%). Two infants showed retarded growth and another developed pneumonia during the course of the treatment.15 So, comparing the different researches with current one, the complications in propranolol therapy though varies from patient to patient but were relatively less common and not a great trouble of tolerance and to manage.
Regarding drug response, the current study results show that, in groups A, excellent responder was of (33%) and they were graded as six, but, in group B only (17 %) of patients had achieved that score. The highest numbers (40%) of respondent in Group B were in category of good responder and their grade was in 4. The mean percent of poor responder (grade 3) in two groups were comparable (10 % ,13% group A and B respectively) , but the mean percent of very poor responder (grade 2) and non responder (grade1) were same (7%) in both groups (p=0.020). This result was also more or less supported by a study where respondents to propranolol were graded as excellent (n= 16.50%), partial responder (n=15.47%) & non responder (n=11.3%).1
Conclusion
In respect to the derived study findings it has been suggested that propranolol therapy could be considered as an emerging and effective treatment over oral corticosteroid therapy for infantile hemangiomas regarding patients' improvement and safety. As the study was carried out only in a single tertiary care hospital of the country with a limited sample size and within a short duration it can be considered as 1st line of treatment replacing the current one (oral corticosteroid). However, a multi centred study with a larger sample size and longer duration is recommended.
References
- Buckmiller LM, Munson PD, Dyamenahalli U, Dai Y, Richter GT. Propranolol for infantile hemangiomas: early experience at a tertiary vascular anomalies center. Laryngoscope 2010; 120:676-81.
- Rizzo C, Brightman L, Chapas AM, Hale EK, Cantatore-Francis JL, Bernstein LJ et al. Outcomes of childhood hemangiomas treated with the pulsed-dye laser with dynamic cooling: a retrospective chart analysis. Dermatol Surg. 2009; 35:1947-54.
- rice CJ, Lattouf C, Baum B, McLeod M, Schachner LA, Duarte AM et al. Propranolol vs corticosteroids for infantile hemangiomas: a multicenter retrospective analysis. Arch. 2011; 147:1371-6.
- Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA et al. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006; 118:882-7.
- agazgoitia L, Torrelo A, Gutierrez JC, Hernández-Martin A, Luna P, Gutierrez M et al. Propranolol for infantile hemangiomas. Pediatr Dermatol. 2011; 28:108-14.
- Book Starkey E, Shahidullah H, Propranolol for infantile haemangiomas: A review. Arch Dis Child. 2011; 96:890-93.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011; 128:e259-66.
- Mendiratta V, Jabeen M. Infantile hemangioma: An update. Indian J Dermatol Venereol Leprol. 2010; 76:469-75.
- Schiestl C, Neuhaus K, Zoller S, Subotic U, Forster-Kuebler I, Michels R et al. Efficacy and safety of propranolol as first-line treatment for infantile hemangiomas. Eur J Pediatr. 2011; 170:493-501.
- Pope E, Chakkittakandiyil A, Lara-Corrales I, Maki E, Weinstein M. Expanding the therapeutic repertoire of infantile haemangiomas: cohort-blinded study of oral nadolol compared with propranolol. Br J Dermatol. 2013; 168:222-4.
- Izadpanah A, Izadpanah A, Kanevsky J, Belzile E, Schwarz K. Propranolol versus corticosteroids in the treatment of infantile hemangioma: a systematic review and meta-analysis. Plast Reconstr Surg. 2013; 131:601-13.
- Klement G, Fishman SJ . Vascular Anomalies: Hemangiomas and Malformations.In: Grossfield Jl, O′Nill,Jr. JA, Fonkalsrud EW , editors.Pediatric Surgery :Mosby Elsevier; Philadelphia publishing. 2006.p2094-110.
- Sans V, de-la-Roque ED, Berge J, Grenier N, Boralevi F, Mazereeuw-Hautier J et al. Propranolol for severe infantile hemangiomas: follow-up report. Pediatrics. 2009; 124:e423-31.
- Hasan M, Rahman M, Hoque S et al. Propanolol for Haeamngioma, Pediatric Surgery International. 2013;29:257-62.
- Chowdhury K M, Hoque S. Management of Alarming Haemanigioma with oral prednisolon. BIRDEM Medical Journal. 2017;7:7-10.
- Hoque S, Das BK and Abid R. Vascular anomalies in children;-By Asian Color Printing, ed. Feb. 2011, P-27.